-40%
Dental Universal Implant Torque & 12 Drivers Wrench 2 Heads Adjustment Handpiece
$ 95.03
- Description
- Size Guide
Description
无标题文档Description
Description:
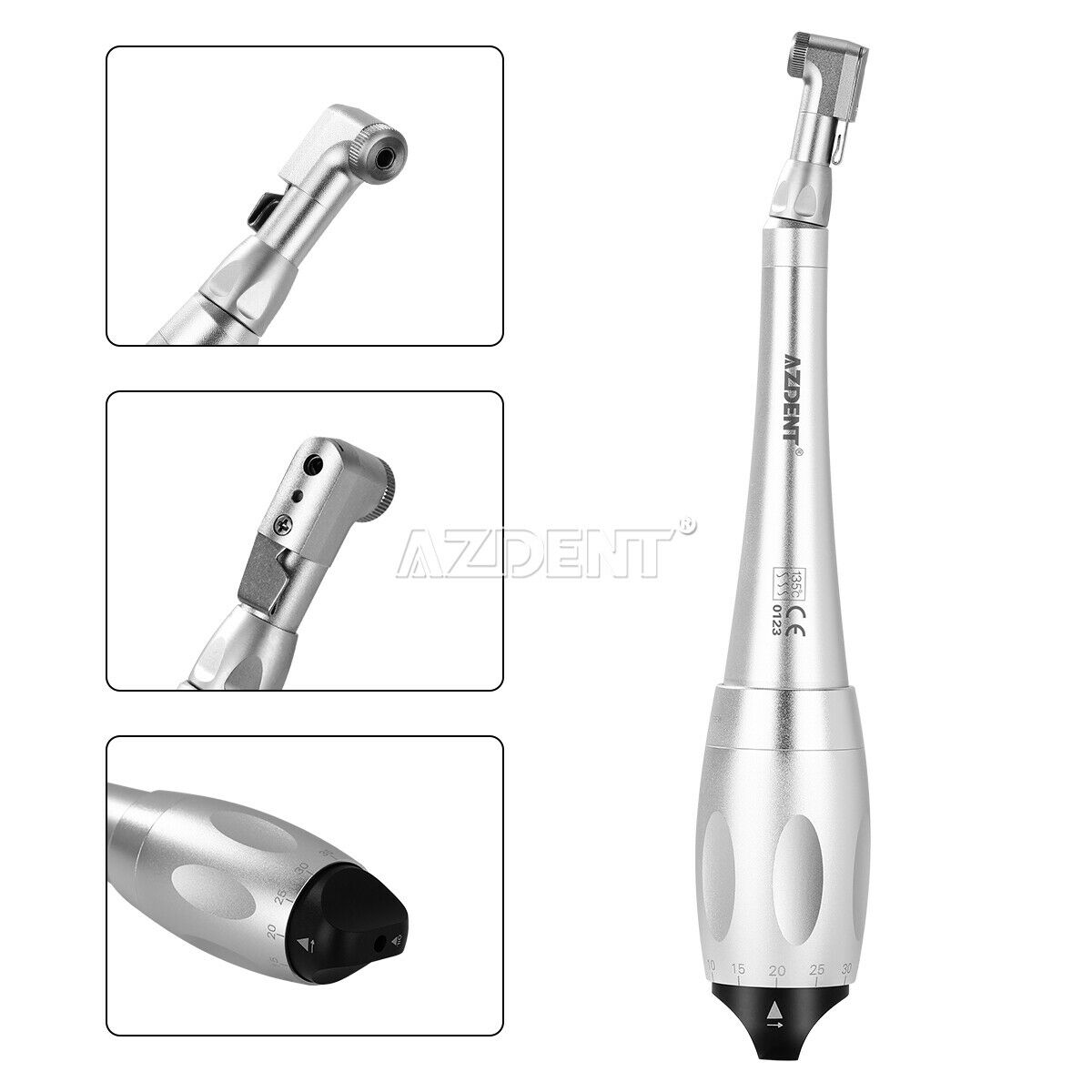
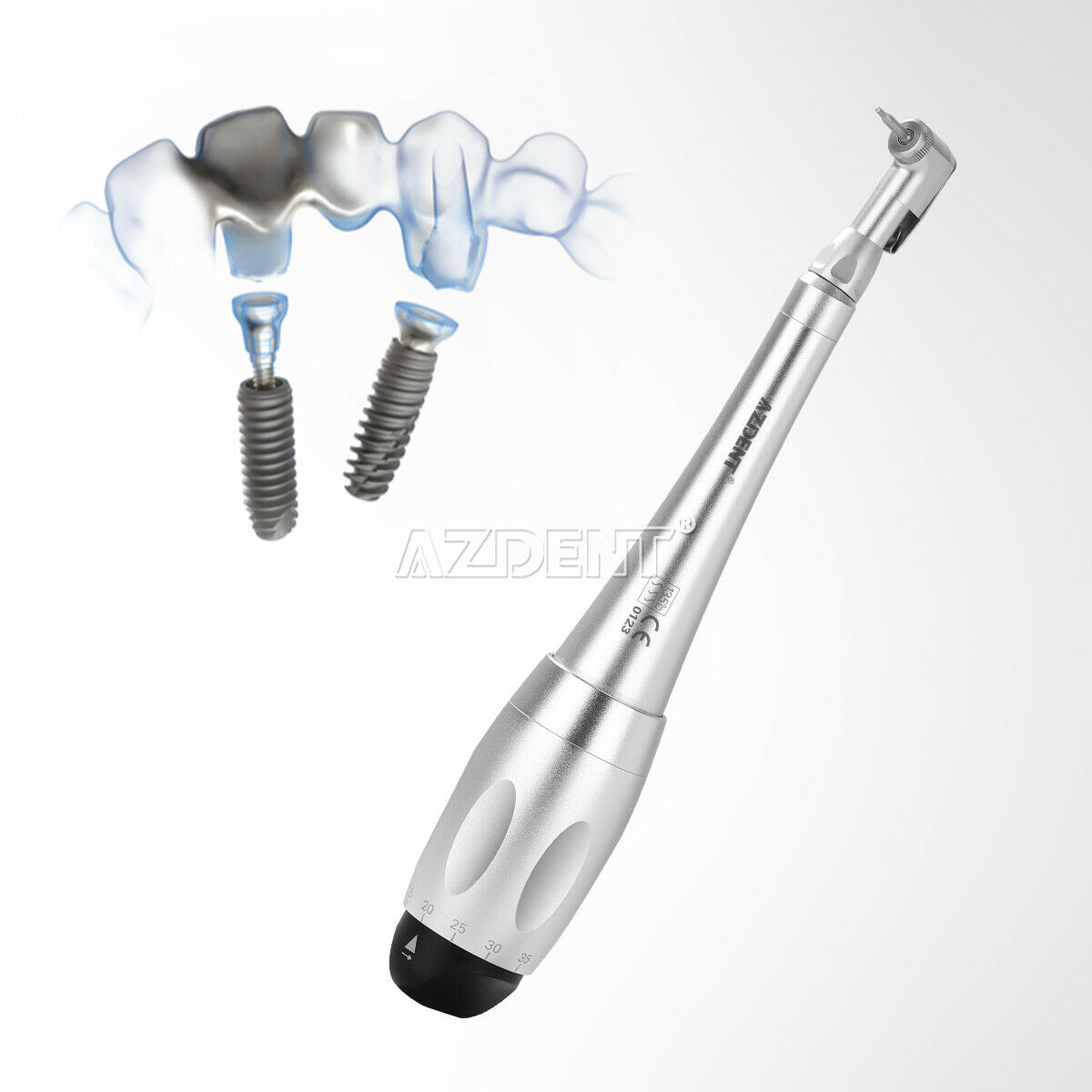
1. Precision instrument equipped with a torque adjustment system for screwing prosthetic components.
2. 7 torque levels (5 to 35 N.cm) and universal connection of the prosthetic mandrels.
3. Micro head and ergonomic handle.
4. Reduced amplitude of movement compared with tightening using a ratchet wrench.
5. Only 160g for a better freedom of movement.
6. Universal torque wrench, recommended with most type of implants.
7. 135°C Autoclavable.
Warranty:1 Year
Drivers:(shank diameter 2.35mm)
0.9mm-S&L
1.0mm-S
1.2mm-S&L
1.25mm-S&L
1.4mm-S
NOB--S&L
ITI ---S&L
The success of the implant treatment depends on the precise tightening of the parts placed directly on the implant.
A pre-stressed tightening of the screw will help avoid any risk of screw loosening.
Also, high tightening torques may lead to screw fracture.
A calibrated tightening can only be guaranteed through the use of a precision instrument offering a torque control system.
The dynamometric manual wrench has been specially designed to meet those requirements.
Package:
Wrench Body *1pc
Spare Latch Type Head *1pc
Drivers *12pcs
K181691
Trade/Device Name: High-speed air turbine handpiece / Low-speed air turbine handpiece
Regulation Number: 21 CFR 872.4200
Regulation Name: Dental Handpiece and Accessories
Regulatory Class: Class I, reserved
Product Code: EFB, EGS
Dated: April 9, 2020
Received: April 27, 2020
FDA Statement
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, you can bid on this item only if you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item.
Seller name:Dentalbox
City,State:SHENZHEN,China
Telephone number:0755-23193580
Relevant Policy